Researcher Profiles

Leonard I. Zon, M.D.
2023 Funding recipient
Using a Quality Assurance Mechanism for Stem Cells to Find Potential Therapies for MDS
Discovery Research Grant 2023
PROJECT SUMMARY
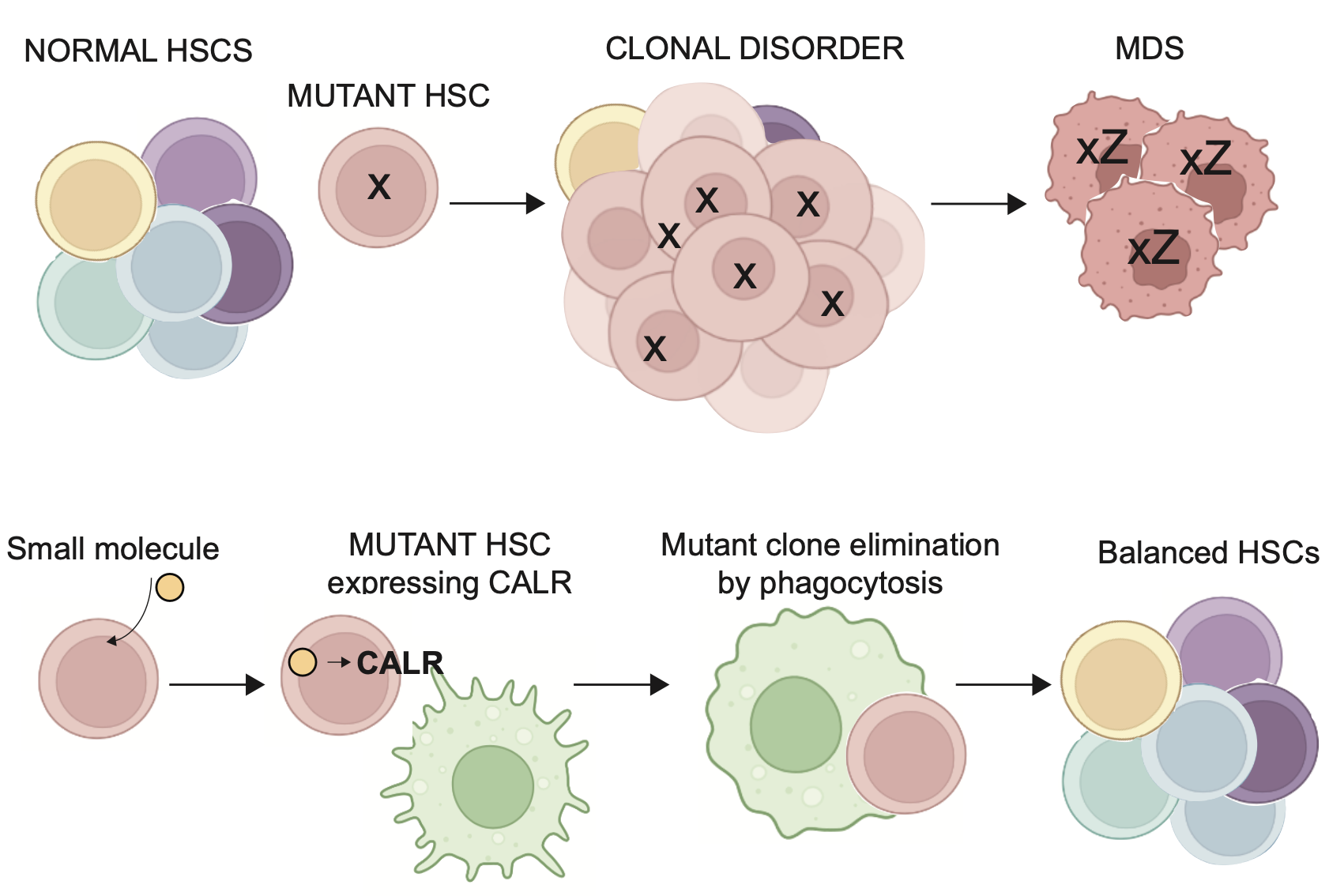
In many adult tissues, the maintenance of organ function depends on stem cells. Although there is a requirement of stem cells for tissue repair, it is unknown if the stem cells are quality assured in any manner. In this study, we will study a new quality assurance mechanism for blood stem cells (HSPCs) in vivo and this may have implications for a target treatment of myelodysplastic. syndromes (MDS). Macrophages interact with HSPC during development through two independent behaviors. A macrophage may completely engulf (“eat”) a stem cell or may scan the stem cell and capture small pieces of it. In the latter situation, the stem cell goes on to divide. This interaction is mediated by the “eat me” signal calreticulin (CALR) on the surface of HSPCs. Macrophage depletion led to unbalanced HSPC diversity in the adult marrow, suggesting that a quality assurance mechanism for stem cells is operative. We plan to define the signals triggering CALR expression on the HSPC surface mediating the removal or amplification of the stem cells. We envisioned that surface CALR is sensed by the macrophages to control the stem cell quality and thought to identify the quality control “code” which is surveilled by the macrophages. We anticipate that understanding the molecular cues underlying the surface CALR expression and its outcome will allow us to use HSPC-macrophage interaction to directly target abnormal HSPCs in MDS. This will prevent the establishment of a malignant clone. We propose to identify candidate pathways that may be underlying the proposed quality control assessment by taking advantage of the surface CALR levels on the stem cell membrane and perform a chemical screen on human cells to find pathways that enhance surface CALR on hematopoietic stem cells. We will validate these chemicals in zebrafish by adding small molecules and monitor the interaction of macrophages and HSPCs in vivo. To further evaluate the treatment specificity that mediates macrophage and HSPCs interaction through CALR we will validate our findings using an in vitro approach, probing CALR expression on the HSPCs membrane and their ability to interact with macrophages. We also will undertake a gene knockout-based screen in hematopoietic cells to evaluate pathways required for surface CALR. These studies will be followed by genetic studies, biochemistry, and chemical genetics to probe the pathways that affect the quality assurance of stem cells. Finally, we plan to study if these chemicals have selected induction of surface CALR on common affected genes in myeloid disorders, namely, ASXL1 and DNMT3A, mutant clones compared to wildtype clones. We propose that understanding the molecular cues underlying the surface CALR expression and its outcome will allow us to directly target abnormal HSPCs clones in clonal hematopoiesis and MDS for macrophage removal.