Researcher Profiles

Richard A. Voit, M.D., Ph.D.
2023 Funding recipient
Uncovering therapeutic vulnerabilities in MDS through investigation of MECOM activity
EvansMDS Young Investigator Award
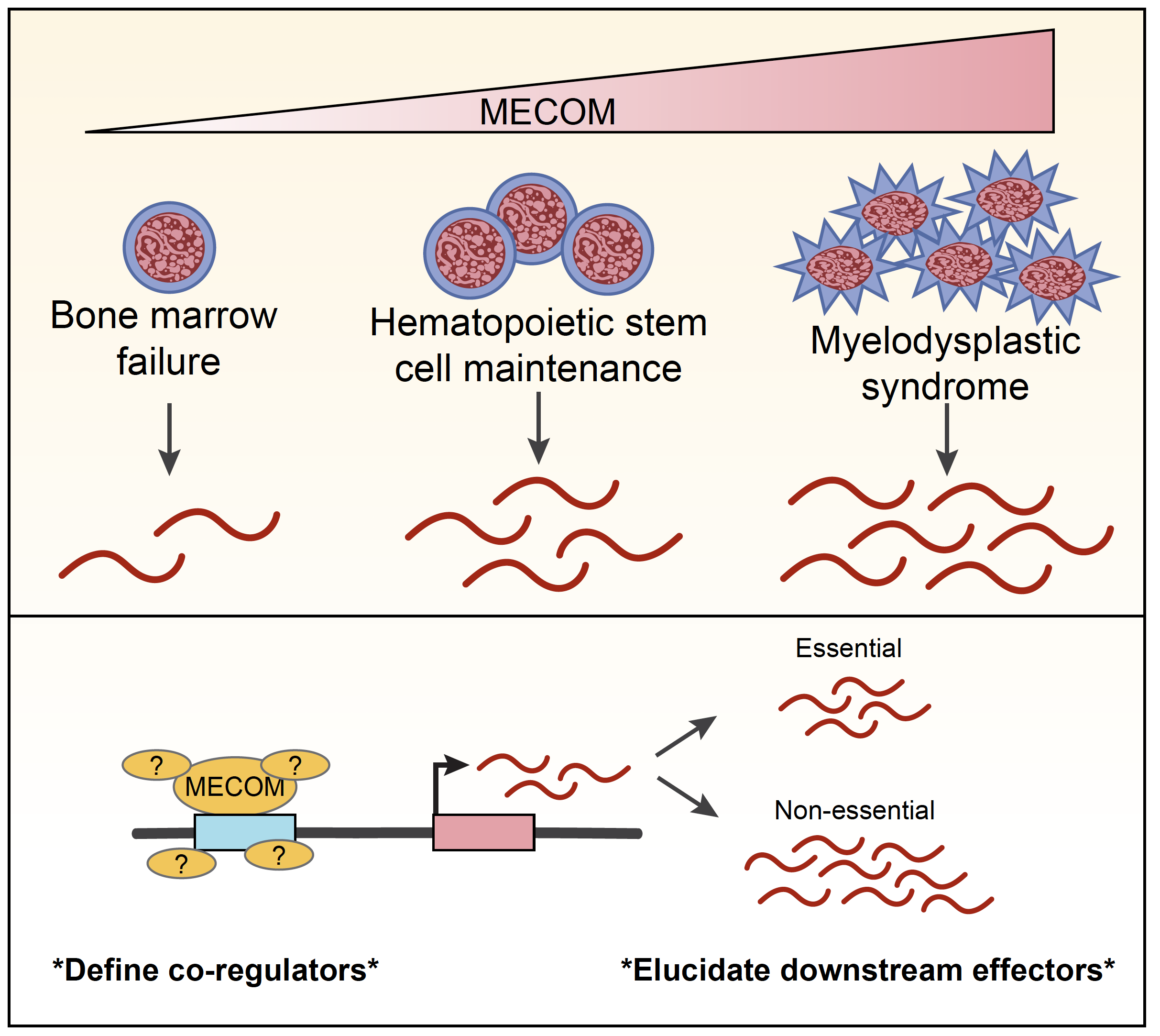
PROJECT SUMMARY
Human blood stem cells produce more than 500 billion new blood cells per day and this process must continue for 8 decades or longer, requiring preservation of the stem cells themselves to support this massive blood cell production. Precise molecular signals are necessary to maintain this balance of stem cell preservation and blood cell production and when these signals are disrupted blood cell production can either be irreversibly shut down in a condition known as bone marrow failure, or be hyperactivated as in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). My prior work showed how a gene called MECOM is crucial to regulate the expression of an important set of several hundred genes that collectively maintain this balance – not enough MECOM causes bone marrow failure and too much MECOM leads to MDS and AML. In addition, MDS with high levels of MECOM is a particularly aggressive form of the disease, and there are no current treatments that specifically interfere with MECOM activity. In this proposal, I seek to better understand how high MECOM expression leads to MDS by looking at two aspects of MECOM signaling. First, I will use precise and synchronized destruction of the MECOM protein in AML cells to uncover the immediate cellular and molecular consequences of MECOM loss. In this way, I will describe how MECOM affects the structure of DNA loops and how that structure makes DNA more or less available to interactions with important cooperating regulator proteins. While I will detect changes across the entire genome of AML cells, I will also specifically hone in on two co-factors that I discovered in my previous work to compete with MECOM in the control of these important genes. Second, I will systematically examine each of the genes specifically regulated by MECOM to determine which individual genes are required for MECOM to cause MDS. My initial studies have shown that one of these gene in particular is necessary for the long-term survival of AML cells and high expression of this gene in patient MDS bone marrow samples is associated with a more aggressive disease course. I will investigate the function of this MDS-essential gene and develop specific methods to disrupt its function in a way that could potentially be used as a targeted therapy for MDS patients in the future.