Researcher Profiles

Peter van Galen, Ph.D.
2023 Funding recipient
Dissecting the Role of T Cells in Preventing the Progression of DNMT3A-Mutated Clonal Hematopoiesis
Discovery Research Grant 2023
PROJECT SUMMARY
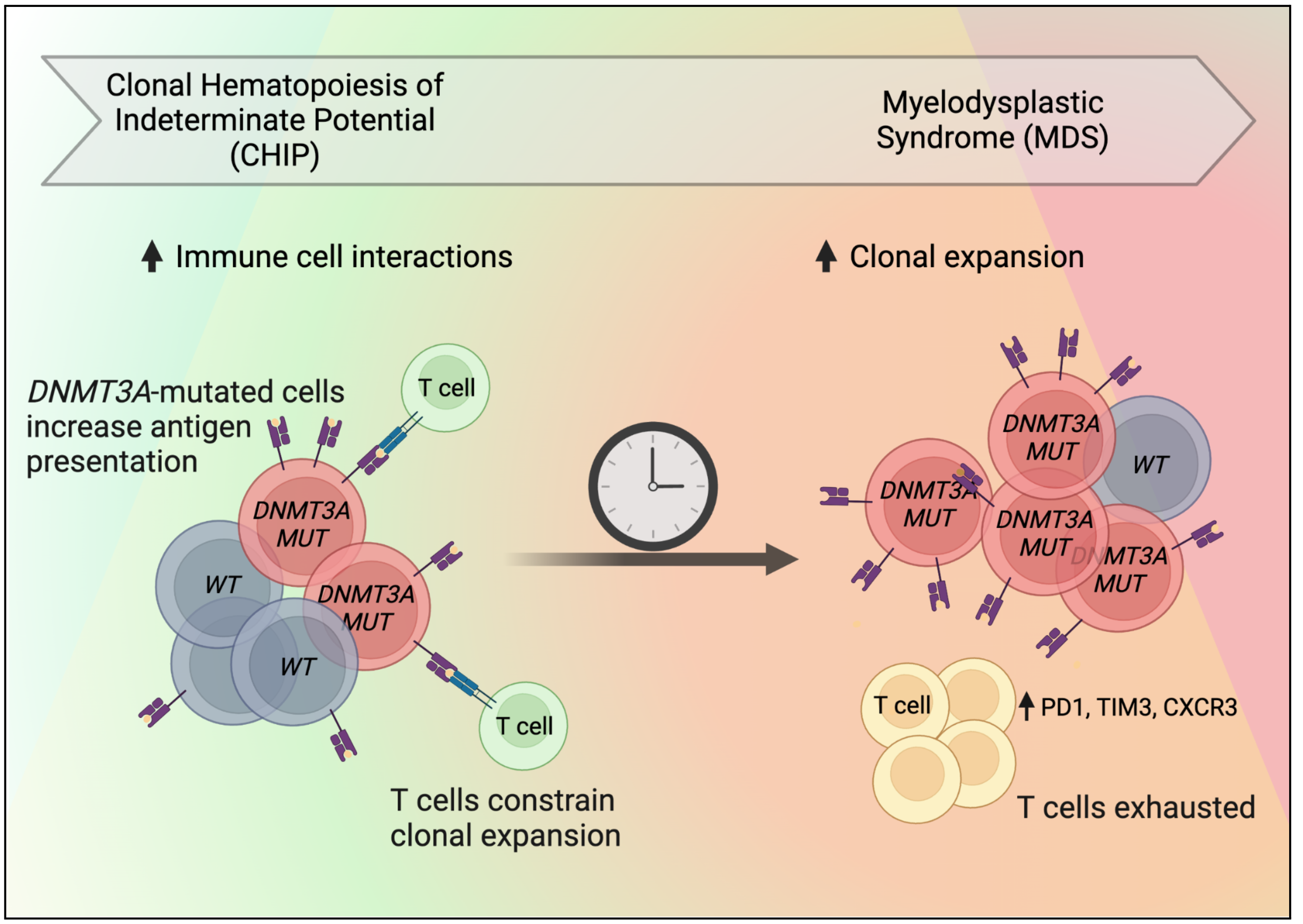
Blood stem cells divide and give rise to all the cell types that circulate in the blood, including various types of immune cells. As we age, blood stem cells acquire mutations in their DNA. Some of these mutations give an advantage by increasing cell divisions. When a blood stem cell acquires such an advantage, it can produce an expanding population of blood cells carrying the same mutation. This is common: more than 10% of people over the age of 65 have mutated cells that can be easily detected in their blood. This condition is termed clonal hematopoiesis of indeterminate potential (CHIP), and it is generally asymptomatic. However, in some people, the mutated cells expand further, which can lead to the development of blood disorders including myelodysplastic syndrome (MDS). The goal of this research is to understand the progression of CHIP into MDS, which is characterized by abnormal blood cells and ineffective blood cell production. If successful, our research can lead to better risk assessment for patients with CHIP, as well as preventative medicine to keep the blood system healthy.
Immune cells can be subdivided into myeloid and lymphoid cells. MDS is characterized by the abnormal production of myeloid cells. Interestingly, there is emerging evidence that lymphoid cells, specifically T cells, also play a role in the progression of CHIP into MDS. An important role of T cells is to eliminate cells with abnormal features. Indeed, experiments in our lab have suggested that T cells target mutated stem cells, which are the cellular origin of MDS. However, we have found that these T cells eventually become exhausted, which may allow the progression from CHIP into MDS. In this project, we propose to work out these interactions between T cells and mutated stem cells. We think the mutated stem cells directly cause T cell exhaustion to their own advantage. If confirmed, this would open up the possibility of helping T cells regain their normal function of eliminating mutated stem cells. This may prevent the progression of CHIP into MDS. To carry out these studies, we will use mouse models and human samples. To create a new mouse model, we have combined tools from cancer biologists and immunologists, so that we can precisely track the interactions between mutated stem cells and T cells in the mice. We also set up a biobank of samples from people with CHIP and MDS. To study these samples, we have developed technologies that allow us to analyze many single cells at once. We will pair these new technologies with computer algorithms to precisely pinpoint which blood cells drive the progression from CHIP into MDS. Overall, we expect that helping T cells eliminate mutated stem cells will restore immune health in older people, especially people with CHIP at risk of progression.