Researcher Profiles

Aaron J. Stonestrom, M.D., Ph.D.
Memorial Sloan-Kettering Cancer Center
2022 Funding recipient
Characterization of the functional and molecular consequences of ASXL1 mutations
EvansMDS Young Investigator Award
PROJECT SUMMARY
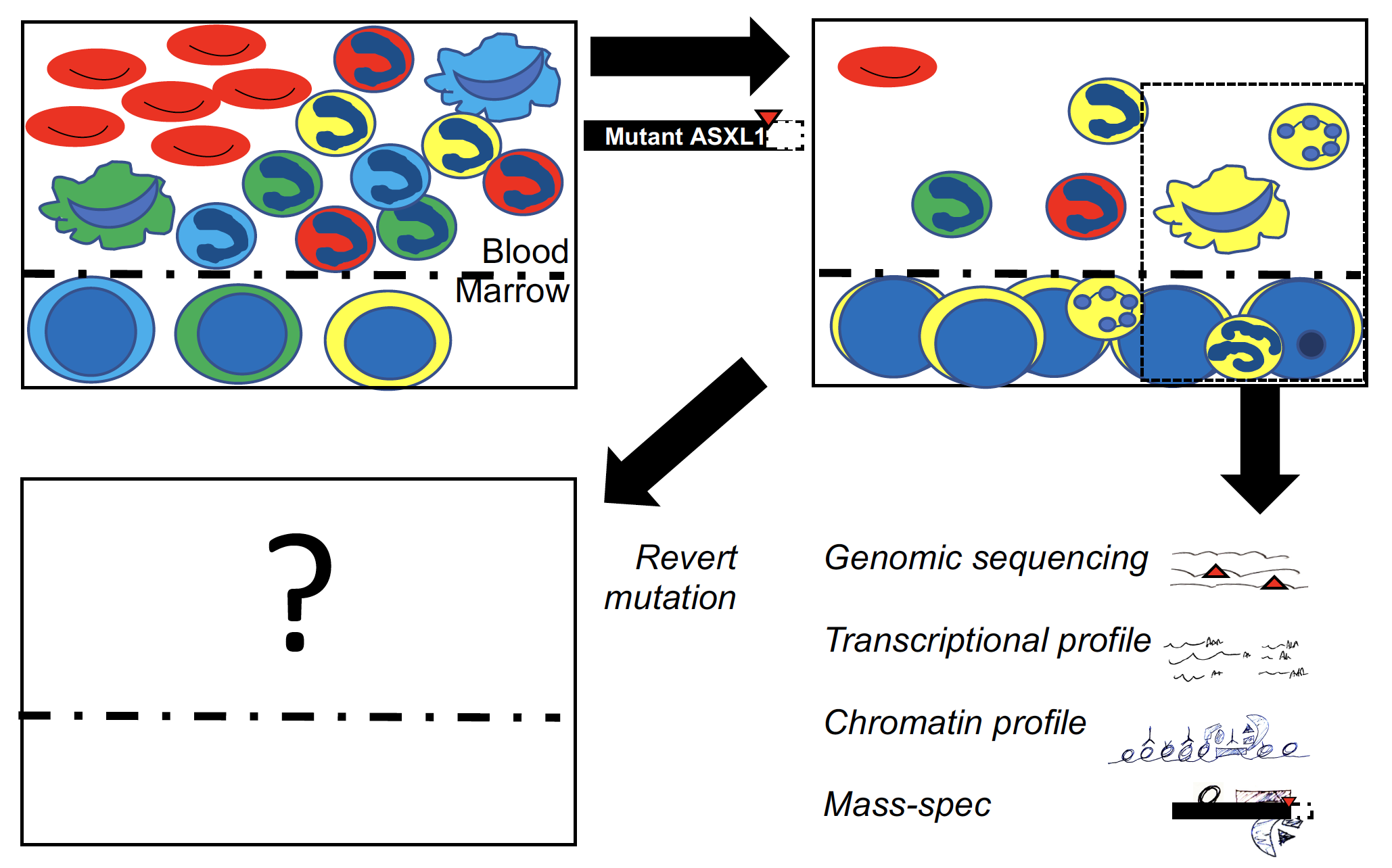
Myelodysplastic syndrome (MDS) develops when abnormal mutant blood stem cell clones take over the bone marrow. In addition to having low blood cell counts, people with MDS are at risk of developing aggressive acute myeloid leukemia (AML). ASXL1 is one of the most commonly mutated genes in many blood stem cell diseases. ASXL1 mutations make MDS more likely to become AML, and AML more difficult to treat. Despite the significance of ASXL1 mutations, we still do not have a clear understanding of their role in MDS. This knowledge deficit presents a major roadblock on the path to better therapies. Our proposal addresses this roadblock by focusing on the scientific question: How do ASXL1 mutations cause clonal blood disease?
This proposal makes extensive use of a genetically engineered mouse model we recently built. This model allows us to control induction of Asxl1 mutations and to control reversion of Asxl1 mutations back to an unmutated state. Our first aim involves first turning on Asxl1 mutations and monitoring how mutant blood stem cell clones evolve. We will look for evidence of additional genetic mutations as well as changes in which genes are activated and how gene activation occurs. We will subsequently turn off Asxl1 mutation at different stages of blood disease development. Asxl1 mutations are likely to be viable therapeutic targets in stages in which mutational reversion stops disease progression. In Aim 2 we will use our engineered mouse model to test whether emerging biochemical ideas about how ASXL1 protein works hold true in a more physiologic setting. We will look for proteins that interact specifically with mutant ASXL1, as well as ASXL1-mutant specific changes in DNA packaging and gene activation.
We have designed our experiments to maximize the potential that they could lead to the identification of therapeutic targets. It is our ambition that performing these studies and establishing these systems will provide a launching pad for future investigations into the biology of ASXL1 and other unmet needs in MDS and related diseases.