Researcher Profiles

Robert Signer, Ph.D.
University of California, San Diego
2025 Funding Recipient
The Role of Proteostasis in Clonal Hematopoiesis and Myelodysplastic Syndrome
Discovery Research Grant 2025
PROJECT SUMMARY
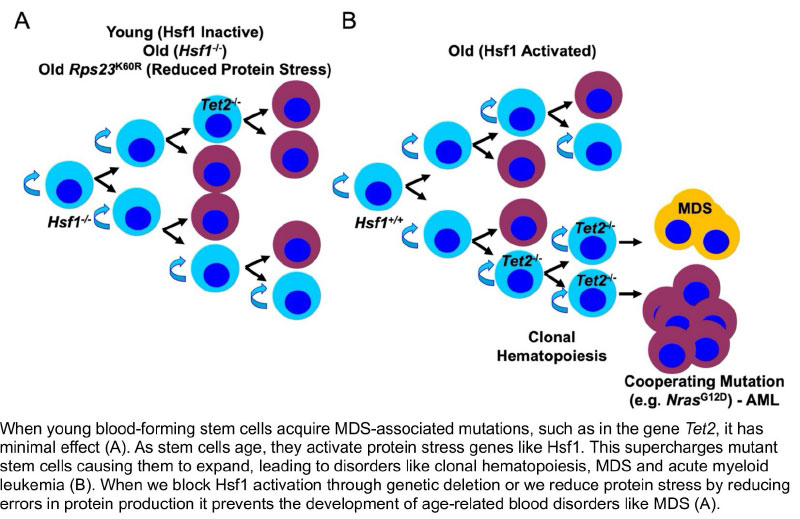
Myelodysplastic syndromes (MDS) are a group of serious blood disorders that often affect older adults. While scientists know that aging increases the risk of MDS, they are still working to uncover exactly why this happens. One key factor that has not yet been examined is a process called proteostasis, which is the body’s protein quality control system, ensuring that our cells stay healthy and function properly by eliminating “protein trash”. As we age, this system starts to fail, leading to the buildup of “protein trash” in blood-forming stem cells in the bone marrow. This buildup may play a key role in the development of MDS.
When stem cells become overwhelmed by damaged proteins, they activate special stress response genes to try and survive. We discovered that one of these genes – Heat shock factor 1 (Hsf1) – acts as a double-edged sword. On one hand, Hsf1 helps healthy stem cells get rid of harmful proteins, keeping them fit and functional as we age. On the other hand, Hsf1 also supercharges precancerous stem cells, allowing them to multiply – leading to a condition call clonal hematopoiesis – a major early warning sign of MDS and other blood cancers.
Our research aims to answer a critical question: Can removing Hsf1 slow down, reduce or even prevent the development of MDS? By understanding how MDS forms, we can unlock new strategies to stop it before it starts.
To take this even further, we developed a new intervention that reduces the production of damaged proteins inside stem cells, lowering their stress levels and keeping stem cells fit without needing to activate stress response genes. This means that aging stem cells will not need to activate Hsf1 as much, potentially preventing harmful changes that lead to MDS and other blood cancers. In this study, we will put our approach to the test, working toward the development of new treatments that could improve stem cell health, slow disease progression, and ultimately offer new hope to MDS patients. If successful, our work could pave the way for better therapies that not only extend life but also improve quality of life for those at risk of or living with MDS.