Researcher Profiles

Rishi V. Puram, M.D., Ph.D.
2022 Funding recipient
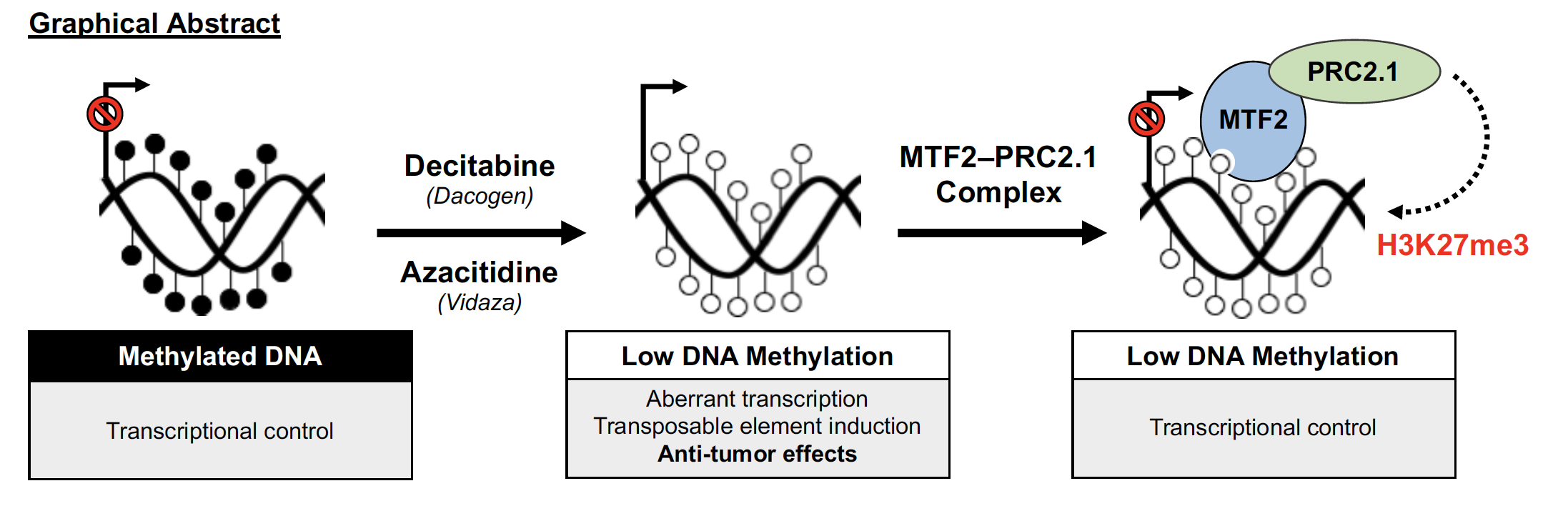
Investigating MTF2-PRC2.1 as a molecular determinant of hypomethylating agent response in MDS
EvansMDS Young Investigator Award
PROJECT SUMMARY
Myelodysplastic syndromes (MDS) are a heterogenous group of bone marrow stem cell disorders characterized by ineffective blood cell production and risk of progression to acute myeloid leukemia (AML). Drugs known as hypomethylating agents, including azacitidine (Vidaza) and decitabine (Dacogen), are cornerstone treatment options for MDS patients. Despite their clinical use for nearly two decades, the anti-tumor mechanism of action of these drugs remains poorly understood. The term hypomethylating agent stems from the ability of these drugs to block DNA methylation, a repressive epigenetic mark involved in gene silencing, but how this targets malignant cells in MDS remains unknown. Using genome- scale CRISPR screening approaches, we unexpectedly identified the gene Metal Response Element Binding Transcription Factor 2 (MTF2) as a critical genetic modifier of hypomethylating agent response in myeloid disease models. MTF2 is a DNA-binding, methylation-sensitive factor that recruits the Polycomb Repressive Complex 2.1 (PRC2.1) to hypomethylated genomic sites for gene silencing – this may be an important mechanism for maintaining transcriptional control following drug-induced loss of DNA methylation. In our proposal, we will determine whether genetic deletion of MTF2 and additional components of PRC2.1 sensitizes cells to hypomethylating agents in pre-clinical models of MDS. We will also determine the epigenetic mechanisms by which MTF2-PRC2.1 prevents the re-awakening of transposable elements, viral remnants that are abundant in the human genome, upon loss of DNA methylation. This work will improve our understanding of nt anti-tumor mechanism of action and offer insights into the development to new therapeutic strategies target DNA methylation in MDS.