Researcher Profiles

Hai Dang Nguyen, Ph.D.
2023 Funding recipient
Therapeutic targeting of R-loop response pathways in spliceosome mutant MDS
Discovery Research Grant 2023
PROJECT SUMMARY
Myelodysplastic syndromes (MDS) are a heterogenous group of blood disorders characterized by ineffective production of blood cells by the bone marrow. RNA splicing factor genes are frequently mutated in 50 to 60% of MDS patients and are associated with poor survival and high risk of progression to secondary acute myeloid leukemia. High-risk MDS patients have a poor prognosis with a median survival ranging from months to a few years, in part due to the lack of therapeutic modalities. Thus, targeted therapies to fill this critical therapeutic gap in MDS are needed.
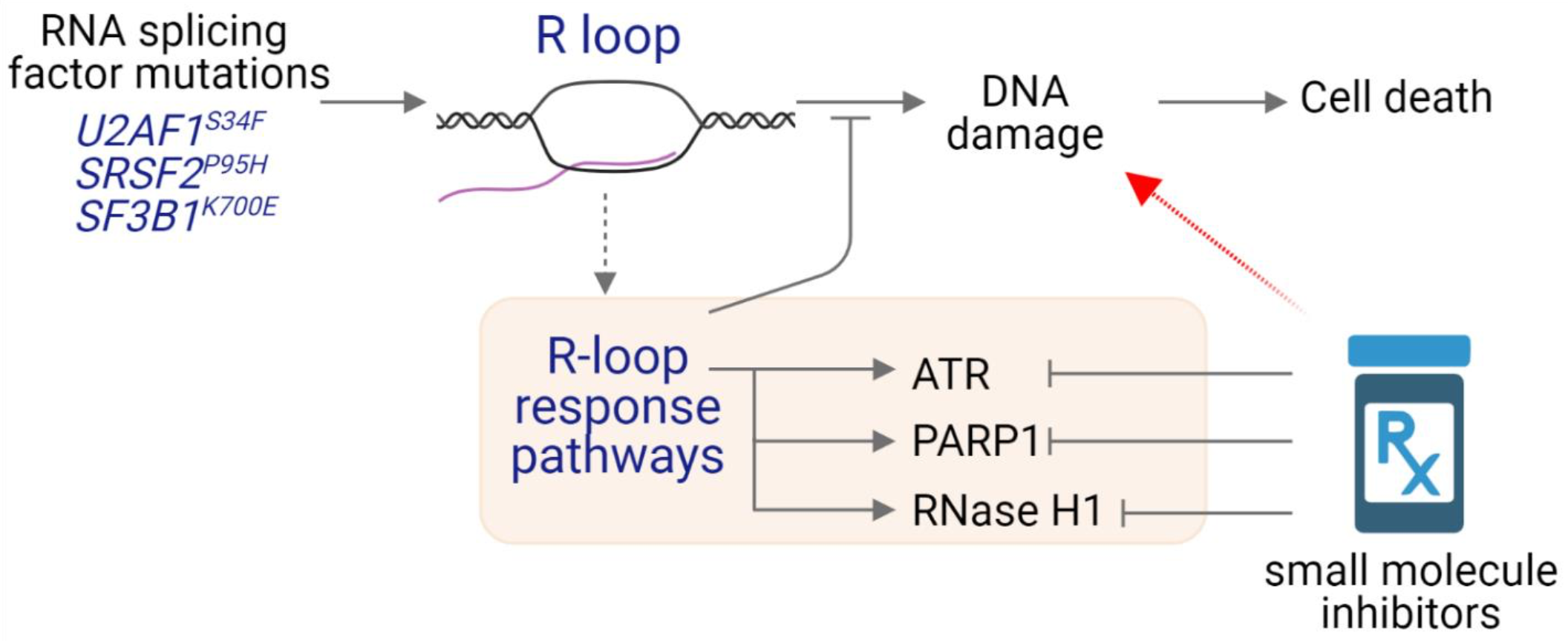
Recent works by us and others showed that perturbations caused by RNA splicing factor mutations can lead to aberrant R-loop accumulation. An R loop is a three-stranded nucleic acid structure containing an RNA:DNA hybrid and a displaced single-stranded DNA. Our preliminary results indicate that olaparib, an FDA-approved inhibitor that targets poly ADP-ribose polymerase (PARP) 1/2 enzymes, selectively kills RNA splicing mutant cells. Our proposed research will provide mechanistic insight into the mechanism of action caused by PARP inhibitors and provide a pre-clinical rationale to repurpose the use of FDA-approved PARP inhibitors in targeted therapy for MDS patients harboring RNA splicing factor mutations.
In this grant application, our overarching goal is to evaluate whether targeting different R-loop response pathways will provide novel therapeutic strategies to treat MDS patients harboring RNA splicing factor mutations in the future (See Graphical Abstract). We propose to determine the molecular mechanisms of PARP inhibitor sensitivity in RNA splicing mutant cells. This will provide molecular insight into PARP1 functions at R loops and new factors in the PARP1 signaling axis that may represent new biomarkers and/or therapeutic targets. Our second aim is to determine whether inhibiting additional R-loop response pathways, such as RNase H1 enzyme, will enhance PARP inhibitor sensitivity in spliceosome mutant MDS. The results from our proposed studies will significantly advance our understand of R-loop biology and provide a rationale to repurpose the use of FDA-approved PARP inhibitors in targeted therapy for MDS patients carrying spliceosome mutations by exploiting the R-loop-associated vulnerability.