Researcher Profiles

Peter G. Miller, M.D., Ph.D.
Massachusetts General Hospital
2023 Funding recipient
PPM1D and replication stress in the development of myelodysplastic syndrome and response to therapy.
Discovery Research Grant 2023
PROJECT SUMMARY
Myelodysplastic syndrome (MDS) is a cancer that comes from blood stem cells that have developed abnormalities in their DNA, termed mutations. These mutant cells grow more aggressively and outcompete normal blood stem cells, and they are unable to generate the normal blood cells we rely on to carry oxygen, form blood clots, and prevent infection. Left unchecked, these mutant cells can also turn into a faster growing cancer called acute myeloid leukemia.
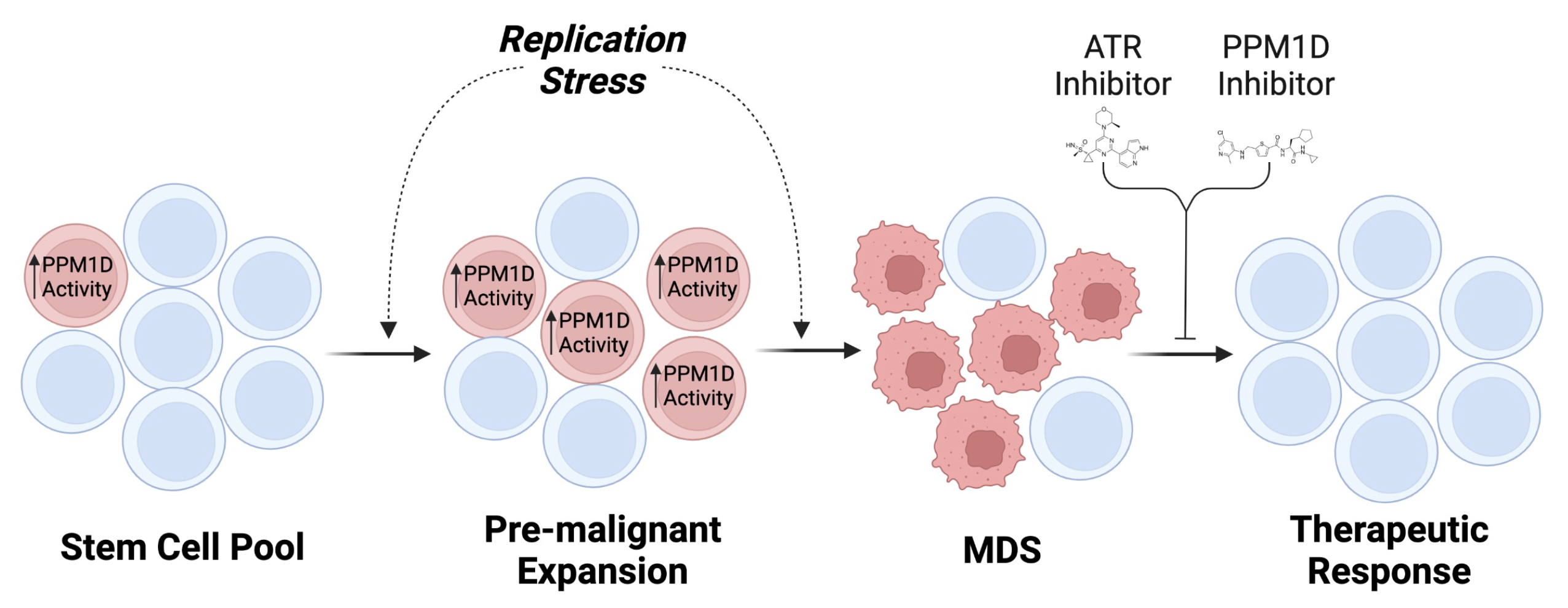
Every time a cell divides it must copy its DNA, and it must do so in a way that minimizes the introduction of any mutations or alterations. Problems that prevent DNA from being efficiently copied are referred to as replication stress (RS). When RS occurs, cells will slow their growth and attempt to fix the DNA errors. If the errors are corrected the cells will continue to divide, but if the RS is not sufficiently addressed by repair mechanisms, the cell will stop growing or actively die to prevent the passage of genome errors to daughter cells. Some cells develop ways to continue to grow despite having high levels of RS, and when this occurs DNA damage can accumulate as the cells divide. This continued damage to the genome can have many consequences, including the transition of pre-cancer cells to cancer cells and the development of treatment resistance in cancer cells.
Our laboratory seeks to develop new ways to prevent and treat MDS. We have identified a gene called PPM1D that is frequently mutated in patients with MDS. Though more common in patients exposed to chemotherapy or radiation for an earlier cancer, PPM1D mutations are also found in previously healthy individuals, suggesting that PPM1D mutations are advantageous for stem cell growth and survival, even before MDS has developed. When we used some new methods to study PPM1D in MDS, we confirmed that PPM1D mutations enhance stem cell growth and survival in either the presence and absence of chemotherapy or radiation, particularly in situations where those cells are undergoing rapid growth and replication. However, the exact way in which PPM1D enhances the fitness of cells in these situations is still unknown, and thus we still need to understand how PPM1D may influence the development of MDS or an eventual resistance to treatments.
Our focus on PPM1D has led to a large and ongoing drug discovery effort to provide new insights into the biology of both normal and cancerous blood stem cells. Based on our own previous studies, we believe that PPM1D increases the activity of both normal and cancerous blood stem cells by allowing cells to tolerate higher levels of RS. We think that PPM1D may contribute to the development of MDS and therapy resistance by enhancing blood stem cells’ ability to grow and survive in the presence of RS. In partnership with other members of the MDS research community, we propose to test this hypothesis using a combination of experimental models and primary human samples. First, we will use our animal models to determine how PPM1D alters blood stem cell growth and survival. Second, we will determine how levels of RS, and the blood stem cell response to it, are influenced by PPM1D. Finally, we will study how PPM1D and RS influence the efficacy of Ceralasertib, a drug in development for MDS.
Along with our collaborators, we aim to improve the health and longevity of patients suffering from MDS. We believe that the efforts outlined in this proposal to understand the relationship between PPM1D and RS will uncover fundamental mechanisms that explain how pre-cancerous cells develop into MDS and progressively become more resistant to treatment. We plan to use what we find to enhance existing clinical approaches—and develop new ones—to preventing the development of MDS and making MDS cells more sensitive to anti-cancer therapies.