Researcher Profiles

James Manley, Ph.D.
2025 Funding Recipient
Targeting metabolic vulnerabilities in SF3B1-mutant MDS as a novel therapeutic strategy
Discovery Research Grant 2025
PROJECT SUMMARY
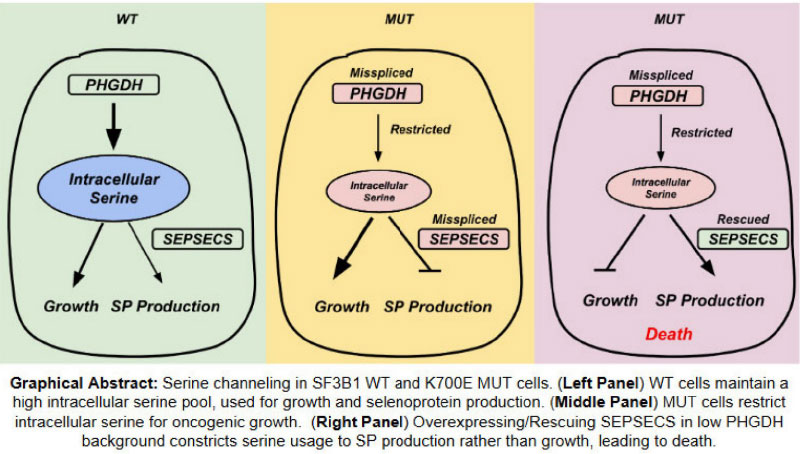
Myelodysplastic syndromes (MDS) are a group of blood disorders in which the bone marrow does not produce healthy blood cells. Some patients with MDS have mutations in a gene called SF3B1. While these patients often suffer from severe anemia and require frequent blood transfusions, there are currently no targeted treatments for this condition beyond bone marrow transplantation. Our research focuses on finding new ways to eliminate these mutant cells by targeting their unique metabolic weaknesses. We discovered that SF3B1-mutant cells have specific defects in two interconnected metabolic pathways that process two important nutrients: selenium and serine. Our research will pave the way for new treatments that specifically target SF3B1-mutant MDS cells, improving outcomes for patients and potentially leading to a cure.
Our approach involves three key strategies. First, we will selectively kill SF3B1-mutant cells by increasing the levels of a selenium-processing protein called SEPSECS, which is significantly reduced in these mutant cells as an adaption to restricted serine synthesis. Second, we will develop a type of molecular therapy called antisense oligonucleotides (ASOs) to correct the defect in how the SEPSECS gene is processed, potentially diverting intracellular serine into the selenium-processing pathway and selectively killing the mutant cells by serine depletion. Third, we will reveal the underlying mechanisms of our therapeutic strategies by investigating how these metabolic changes impact overall cell metabolism and growth using specialized laboratory techniques. Our research will pave the way for new treatments that specifically target SF3B1-mutant MDS cells, improving outcomes for patients.