Researcher Profiles

Ravindra Majeti, M.D., Ph.D.
2020 Funding recipient
Characterization and Therapeutic Targeting of Mutant ASXL1 in MDS
Discovery Research Grant 2020
PROJECT SUMMARY
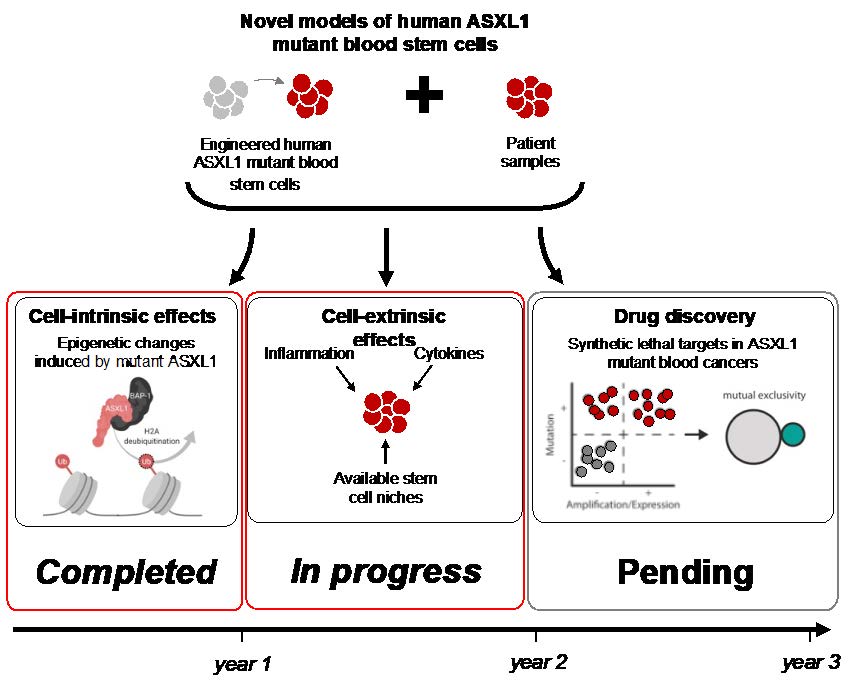
Our understanding of the development of blood cancers in general, and myelodysplastic syndromes (MDS) in particular, has improved markedly over the past decades. Importantly, the finding that specific mutations are sequentially acquired in individual genes has shed light on how blood cancers develop from healthy blood cells. The Majeti lab has contributed to our understanding of blood cancer development by identifying the initiating mutations in blood stem cells, termed pre-leukemic stem cells. Others have subsequently shown similar findings, and importantly were able to show that these initiating mutations can even be found in a significant proportion of healthy individuals, a condition termed clonal hematopoiesis. In these individuals, blood stem cells carrying these initiating mutations can ultimately lead to the development of myeloid malignancies, but in most cases do not. One of the frequently mutated genes in clonal hematopoiesis is ASXL1, which is thought to act as a regulator for how genes are activated. In patients with myeloid malignancies including MDS, the presence of a mutation in ASXL1 is associated with very poor prognosis including poor treatment response. Our understanding of ASXL1 mutations remains limited, most likely due to the lack of representative model systems, and to date, no treatment approaches for blood cancers with mutant ASXL1 have been developed. The goal of this project is thus to:
- Develop a better understanding of how mutations in ASXL1 change the function of the protein
- Determine whether external stressors such as inflammation or the availability of stem cell niches contribute to the development of ASXL1-mutant malignancies
- Determine whether a computational approach developed in the Majeti lab based on synthetic lethality might yield promising therapeutic targets for ASXL1-mutant malignancies
Over the past year, we have begun to explore the influence of external stressors on clonal expansion and the development of ASXL1-mutant malignancies. To interrogate this, we leveraged our model system of using primary human blood stem cells from healthy donors to introduce mutations in ASXL1 using CRISPR/Cas9. Using this system, we previously demonstrated key cell-intrinsic elements of mutant ASXL1 biology.
When exposed to inflammatory mediators, we observed an increased retention of stem cell features within human mutant ASXL1 hematopoietic stem and progenitor cells. The difference between mutant and wildtype HSPCs in our assays was greatest under the influence of the TLR3 ligand PolyI:C, a synthetic analogue of double-stranded RNA that is frequently used to mimic viral infections experimentally. In addition, we also interrogated whether hematopoietic stem and progenitor cells with mutant ASXL1 show differential migration towards factors associated with stem cell niches. Here we show that functional hematopoietic stem and progenitor cells with mutant ASXL1 show increased migration towards the niche factor CXCL12 in vitro. Both of these aspects have thus far been interrogated using our primary human hematopoietic stem cell system in vitro and we are actively expanding these experiments while also performing mouse experiments to address these questions in vivo. Taken together, our work to date on the as illuminated key elements of mutant ASXL1 biology and will serve as a foundation for the remaining goals.