Researcher Profiles

Bin Lu, Ph.D.
Memorial Sloan-Kettering Cancer Center
2023 Funding recipient
Mapping the distribution and function of R loops in MDS at unprecedented resolution
EvansMDS Young Investigator Award
PROJECT SUMMARY
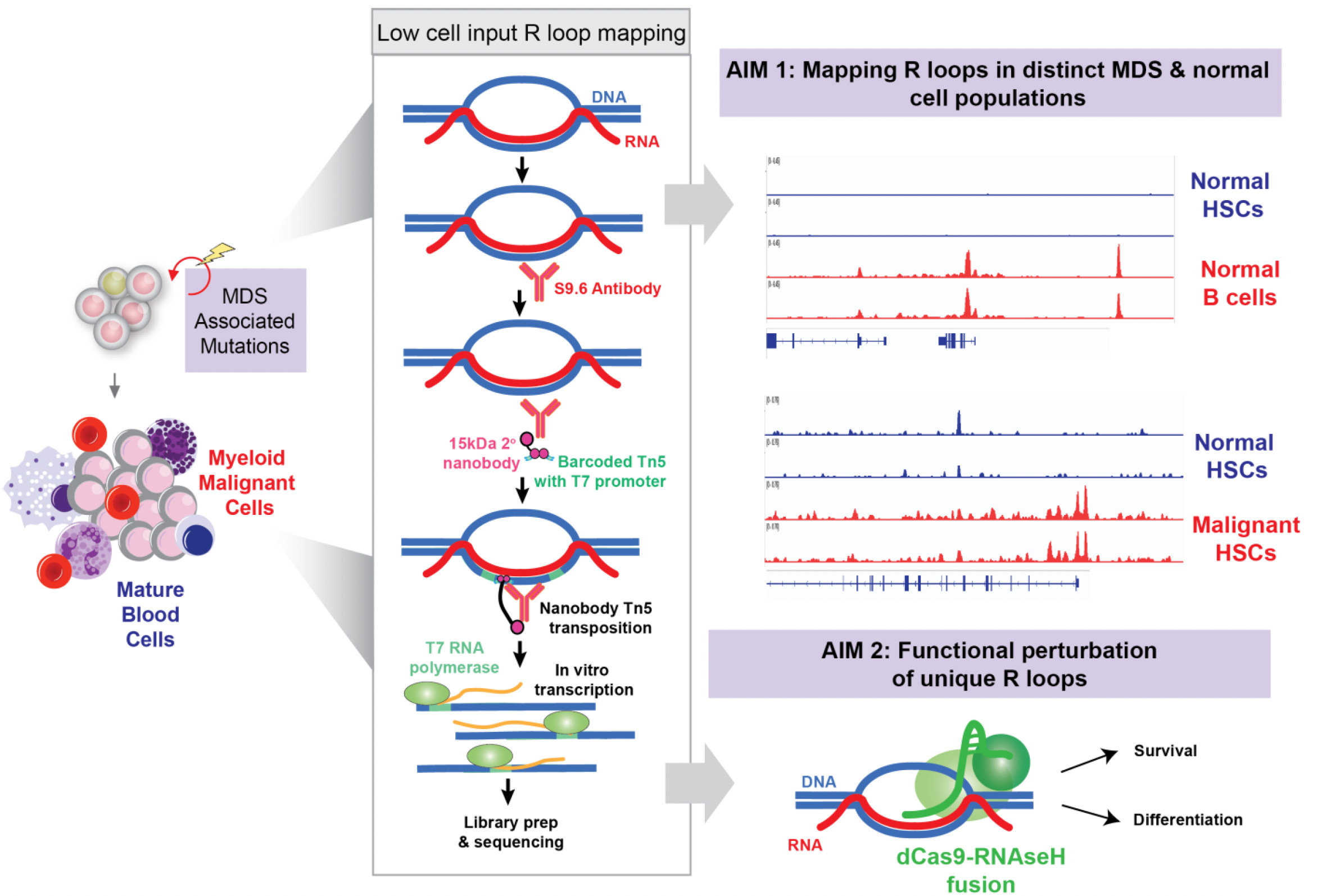
Increased understanding of the genetic causes of myelodysplastic syndromes (MDS) has identified that many gene mutations that are acquired in life and drive development of MDS promote the formation of so- called “R loops.” R loops are structures where RNA, which is produced from the double-stranded DNA molecule, is bound to one strand of DNA while leaving the other strand of DNA displaced. R loops are important as they can serve as sites for DNA damage and also can regulate the patterns of genes expressed in cells. The discovery of increased R loop formation in certain populations of bone marrow cells from patients with MDS has led to clinical trials of drugs targeting removal of R loops as a way of eliminating MDS cells with elevated R loop abundance.
Despite these advances identifying increased R loop formation in MDS patients and the use of drugs to target R loop metabolism in MDS, we still do not have a clear understanding as to what role R loops play in MDS disease development. This limitation is, in part, the result of an inability to measure R loops in precise detail in patient samples due to small cell numbers obtained from patient biopsies. To this end, I have recently developed a new technique to measure R loops in very small cell numbers, and even in individual cells using sequencing technologies. This technique can measure the amount of R loops and their locations in precise genomic detail. This technology has enabled us, for the first time, to measure R loops and their locations in specific cell types such as normal blood stem cells and MDS disease stem cells.
We have now used these techniques in blood stem cells and mature blood cells from mice as well mouse models of acute myeloid leukemia (AML). This effort revealed striking differences in the types of R loops between AML and normal cells as well as unique R loops in each distinct cell type. We now plan to perform similar studies in MDS patient samples and normal human bone marrow cells. Furthermore, we plan to study the actual function of individual R loops to determine how they contribute to MDS development and if their elimination can serve as a new potential therapy for MDS.