Researcher Profiles

Isaac López-Moyado, Ph.D.
La Jolla Institute for Immunology
2025 FUnding Recipient
Inhibition of RNase H enzymes to increase R-loops: a new therapeutic strategy for myelodysplastic syndromes
EvansMDS Young Investigator Award 2025
PROJECT SUMMARY
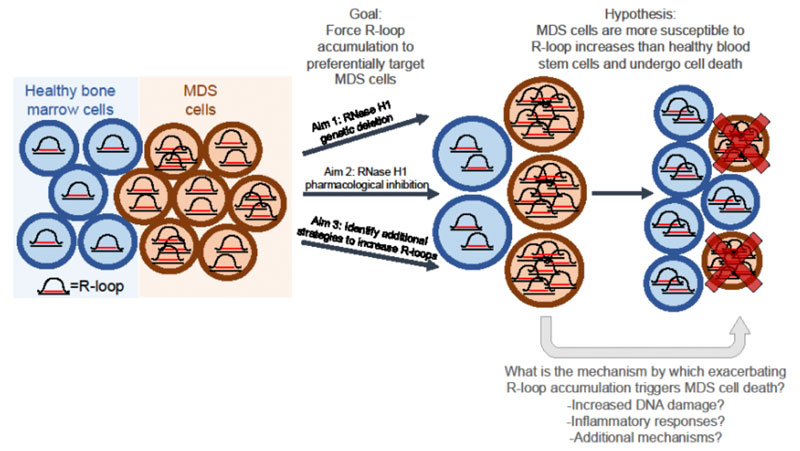
Cancer cells often display aberrant accumulation of RNA-DNA hybrids known as R-loops. R-loops form in healthy cells during many physiological processes, such as transcription and DNA repair, but aberrant accumulation of R-loops, as observed in myelodysplastic syndromes (MDS), can be a source of DNA damage and inflammation. My recent research has shown that in models of myeloid malignancies driven by TET mutations, cancer cells display R-loop accumulation, and that further exacerbating R-loop accumulation by genetic deletion of R-loop-suppressor RNase H1 impaired cancer cell proliferation. Given these preliminary results, I hypothesize that elevated R-loops are an “Achilles heel” of certain subtypes of MDS, and that impairing RNase H1 activity to further increase R-loops and DNA damage in cancer cells might be a reasonable and broadly applicable strategy in the treatment of MDS. This hypothesis will be addressed in the following Aims: (1) characterize the effects of RNase H1 loss in MDS cells with and without TET2 mutations; (2) test if pharmacological inhibition of RNase H1 impairs the proliferation of myeloid cancer cells; (3) identify additional R-loop suppressors whose silencing will impair the proliferation of malignant myeloid cells. We anticipate that increasing R-loops in myeloid cancer cells, by inhibiting RNase H enzymes and potentially by novel approaches discovered within this application, will constitute a new therapeutic strategy for MDS treatment.