Researcher Profiles

Stavroula Kousteni, Ph.D.
Columbia University Medical Center
2022 Funding recipient
Assessing the Role of SAA Inflammatory Signaling in Age-associated MDS Initiation and treatment
Discovery Research Grant 2022
PROJECT SUMMARY
Myelodysplastic syndromes (MDS) comprise a variety of blood cancers characterized by ineffective hematopoiesis: inability to form blood cells. In MDS patients, immature progenitors of blood cells do not mature properly leading to anemia and compromising immune responses. Interestingly, as we age, several alterations in these progenitors or their surrounding environment in the bone marrow (BM microenvironment or niche) where they grow and mature can perturb the regular hematopoiesis process. Indeed, one of the main defining risk factors of MDS is age (median age at diagnosis is 71 years), and MDS is commonly seen after 60 years of age, with a gradually increasing overall incidence in the past 30 years as the overall population age. This year in the US alone, ~15,000 people will be diagnosed with MDS, and ~30% of them will progress to acute myeloid leukemia (AML), making MDS and AML one of the most common blood disorders.
Despite intense research in understanding the complex molecular mechanisms underlying MDS over the past 10-15 years, clinical management has barely changed for the last half-century. Although a variety of complex and personalized therapeutic approaches exist for MDS, yet they are mainly noncurative and aimed mainly at improving quality of life and delaying disease progression. Stem cell transplantation remains the only potentially curative option; however, is only accessible to a small number of fit patients (below the age of 75 and otherwise healthy). Moreover, current treatments have focused on targeting the cancer cells that give rise to the disease, but the cancer will eventually mutate to evade the drugs used and relapse will occur. These limitations highlight the urgent medical need for more basic research that can translate into clinical trials in well-defined subgroups of MDS patients.
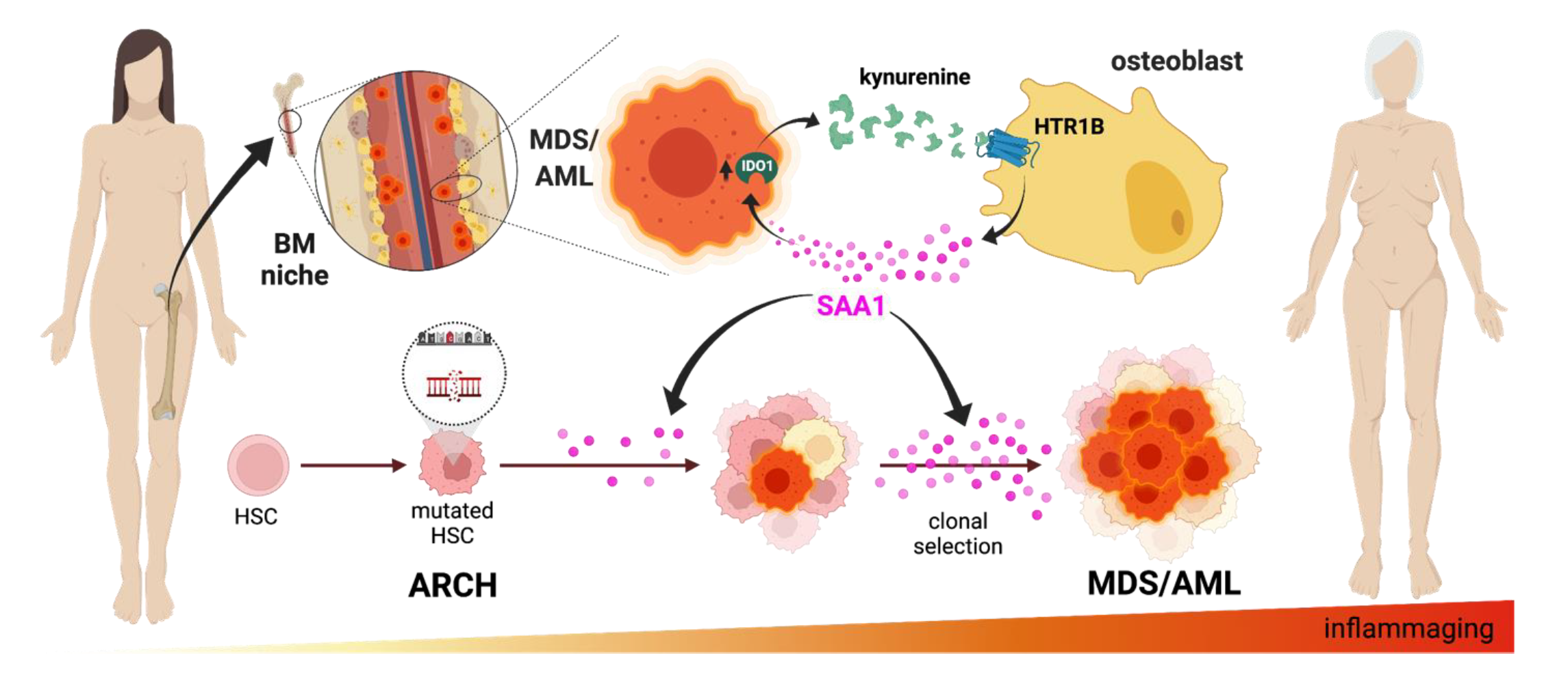
We now know that the BM niche, acts as an ecosystem for disease progression, playing a crucial role in the resistance to therapy. Moreover, cancer cells remodel this niche to their own advantage. Thus, instead of directly targeting the cancer cell, turning a friendly microenvironment for cancer cells into a hostile one, may be an effective treatment strategy.
We have found a mechanism of communication between MDS cells and osteoblasts, the bone forming cells. MDS cells instruct osteoblasts to secrete an inflammatory molecule Serum Amyloid A1 (SAA1). In turn, SAA1 acts on MDS cells to promote their growth. SAA1 levels are increased in the BM of MDS patients as compared to healthy individuals of the same age, and increase further as the disease progresses from MDS to AML, indicating a possible use as biomarker in the clinic. Moreover, SAA1 levels within the BM increase with aging in healthy persons. Our hypothesis is that SAA1 can be used as a market to detect and monitor the appearance of MDS and its progression, and may help stratify the patients to inform intervention and prevent disease progression.
Our goal is to target and inhibit this inflammatory signal within the BM niche. This cancer-cell independent approach used as a standalone intervention or in combination with chemotherapy, could overcome resistance, prevent disease progression and be used as a maintenance therapy to prevent relapse.