
Researcher Profiles

Clara Kielkopf, Ph.D.
University of Rochester, Center for RNA Biology
2014 Grant Recipient
Molecular Consequences of Prevalent U2AF1 Mutations in Myeloid Neoplasms and Lung Adenocarcinomas
Basic Science Research Grant 2014
PROJECT SUMMARY
Specific mutations in the U2AF1 proto-oncogene are prevalent among patients with blood malignancies, including 10-12% of patients with myelodysplastic syndrome (MDS) without ring sideroblasts and 8-11% of patients with chronic myelomonocytic leukemia (CMML). Such mutations correlate with more rapid progression to leukemia as well as decreased overall survival. For patients who achieve complete remission after intensive therapy, it has been found that the U2AF1 mutation is lost and the normal gene recovered.
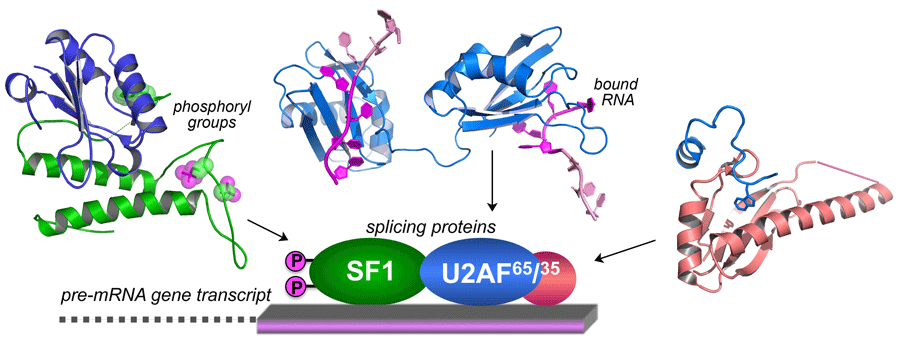
Although defects in gene expression that coincide with U2AF1 mutations have been identified, the molecular cause of these defects remains unclear. Through EvansMDS funding, we discovered that the most common U2AF1 mutation (S34F) alters the protein’s ability to recognize specific sites within a gene’s RNA transcript that signal the process of RNA splicing, which in turn produce different protein products. Specifically, we found that the S34F-U2AF1 mutation typically decreases the protein’s ability to bind representative RNA sites designated by a “UAG” sequence and increases binding to “CAG” sites. We leveraged this information to identify small molecules that modulate U2AF–RNA recognition. We are characterizing and optimizing these small molecules to lay groundwork for potential biochemical, and in the longerterm, therapeutic tools.
Beyond key research funds, the annual EvansMDS meetings have strengthened key collaborations forged between our molecular-level research laboratory, and top clinical- and cellular-level research groups. These relationships will provide an avenue to move our results “from-bench-to-bedside”. In this post-genomic era, an individual patient’s own genetic information – including the absence or presence and type of U2AF1 mutation – serves as a diagnostic tool that currently attainable for a modest price. We envision that the results of this proposal help to understand the molecular-level details of MDS and related hematologic malignancies, and provide a forum to validate U2AF1 as an effective target for “Personalized Medicine” in patients afflicted with U2AF1-mutations and related diseases.
PUBLICATIONS
Okeyo-Owuor, T., B.S. White, R. Chatrikhi, D.R. Mohan, S. Kim, M. Griffith, L. Ding, S. Ketkar-Kulkarni, J. Hundal, K.M. Laird, C.L. Kielkopf, T.J. Ley, M.J. Walter, T.A. Graubert, U2AF1 mutations alter sequence specificity of pre-mRNA binding and splicing. Leukemia, 2015. 29(4): p. 909-17.
