Researcher Profiles

Max Jan, M.D., Ph.D.
Massachusetts General Hospital
2023 Funding recipient
Engineering potent and selective immunotherapies of MDS using targeted protein degradation
Discovery Research Grant 2023
PROJECT SUMMARY
Myelodysplastic syndrome (MDS) is a clonal disease caused by the sequential acquisition of genetic mutations in blood forming stem cells, leading to impaired function of the blood-forming system (hematopoiesis). Bone marrow transplantation (BMT) is the only potentially curative approved therapy for people with high risk MDS. In this treatment, the patient’s diseased blood forming system is replaced with a donor’s. Fundamental to this clinical protocol is the transfer of rare donor hematopoietic stem cells, uniquely capable of limitless self-renewal and differentiation to form all blood cell types. BMT is referred to as the original immunotherapy, in which donor immune cell recognition of subtle differences on diseased cells is essential for MDS eradication. Unfortunately, relapse after transplantation is common and carries a poor prognosis. There is a critical unmet need for innovative strategies to prevent and effectively treat relapse after transplantation.
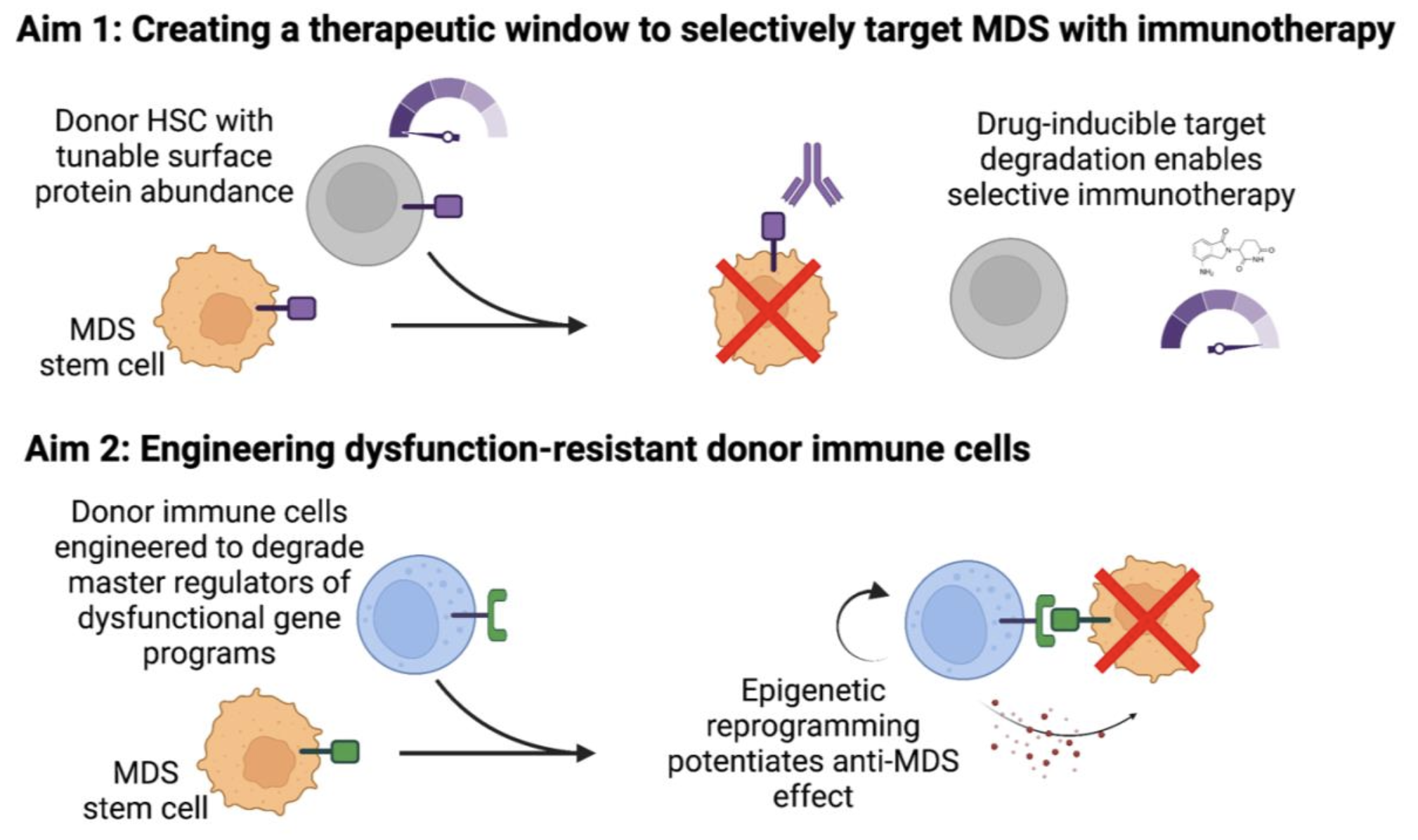
Two fundamental challenges limiting the effectiveness of BMT for MDS will be investigated in this proposal. First, there is a lack of therapeutic window to effectively target MDS stem cells while sparing normal donor stem cells, because these cell types are overwhelmingly similar. Second, donor immune cells encounter complex challenges that in many cases lead to dysfunction and ineffective MDS targeting.
Over more than six decades of clinical experience with BMT, continual refinement and optimization has led to successively improved patient outcomes. Innovations in genome engineering and synthetic biology now enable opportunities for next-generation cell therapy platforms. My laboratory has developed precision, customizable molecular switch technologies to degrade proteins that limit the therapeutic potential of engineered cells. These clinically suitable technologies are composed of human sequences and approved anti-cancer drugs to allow for direct clinical translation. In Aim 1, we will engineer donor hematopoietic stem cells with synthetic circuits to evade MDS-direct immunotherapy, thereby creating a therapeutic window for selective MDS cell eradication and preserved normal hematopoiesis. In Aim 2, we will learn from the clinical experience of exceptional responders to engineer dysfunction-resistant donor immune cells more capable of sustained MDS cell targeting. Once completed, the proposed work will seek to overcome fundamental limitations of immune cell therapy for MDS using emerging synthetic biology approaches, creating investigational gene- and cell-based therapies amenable to direct clinical translation for people with MDS and limited treatment options.