Researcher Profiles

Benjamin L. Ebert, M.D., Ph.D.
2023 Funding recipient
Preclinical models of MDS with multiplexed genetics
Discovery Research Grant 2023
PROJECT SUMMARY
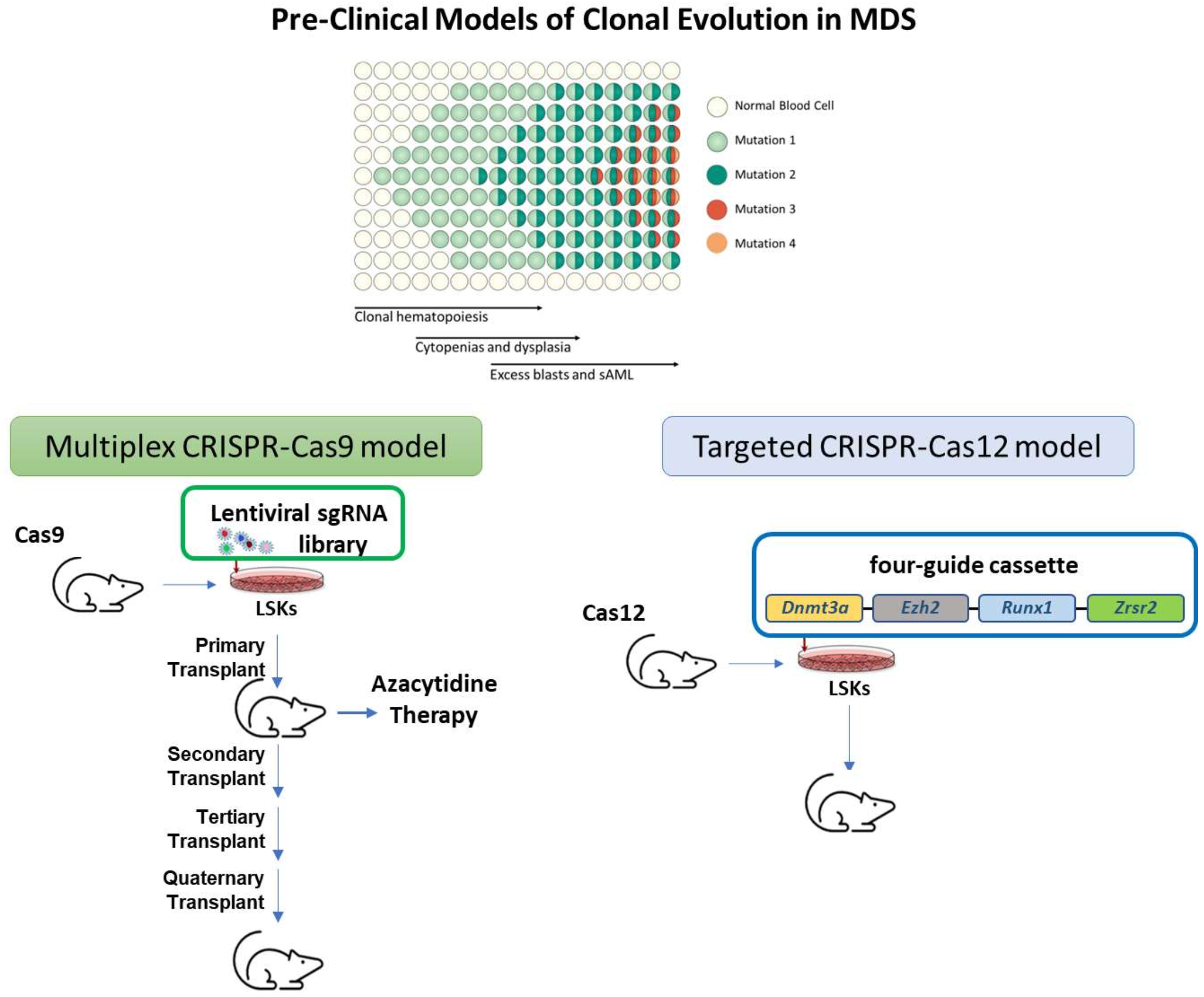
Myelodysplastic syndrome (MDS) is associated with poor survival and limited treatment options. In order to develop new therapies, better pre-clinical models are needed to guide the development of clinical trials. While there are suitable mouse models for some pieces of the MDS puzzle, building a more comprehensive version is challenging due to the complexity of numerous genetic mutations driving the disease and technical challenges that have stymied the development of model systems. In the proposed studies we will integrate multiple technical advances using two different genome editing approaches, CRISPR-Cas9 and CRISPR-Cas12, to build mouse models tailored to suit a complicated disease.
Genetic drivers of MDS transform precursor cells that live and transform in a complex microenvironment in vivo. In general, MDS is driven by the combination of multiple genetic changes, involving 4 or more genes mutated at once in a single MDS blood cell. These cells evolve over time, with each originating cell generating “clones”, groups of similar cells which may dominate or die depending on the characteristics of the clone.
In prior studies, we have created in vivo models using CRISPR-Cas9 to insert a library of genetic changes into cells that have been observed in humans with MDS. Since mutations are inserted randomly, this produces a variety of changes, either singly or in combination, but this allows heterogeneity play out in vivo. The clones compete and then expand or contract over time. By serially transplanting the resulting populations we can observe interactions and mechanisms of cooperation. As an extension, we and others have used similar gene editing approaches to introduce mutations into human precursor cells which are then transplanted into immunodeficient mice. We have also created genetic labels, barcoded CRISPR guide RNAs, which allow us to track and characterize clonal evolution.
CRISPR-Cas12 gene editing then allows us to recreate the most promising combinations of genes, introducing mutations into 4 or more genes in a single cell. We will leverage these approaches to understand clonal evolution following bottlenecks imposed by serial transplantation or azacytidine therapy. Ultimately, the hope is that multiplexed editing of genes will provide better mouse models, and that this will enable robust preclinical studies to guide effective clinical trials.