Researcher Profiles

Simona Colla, Ph.D. 2023
University of Texas MD Anderson Cancer Center
2023 Funding recipient
Targeting the EIF2AK1 signaling pathway to overcome ineffective erythropoiesis in myelodysplastic syndrome with ringed sideroblasts and SF3B1 mutations
Discovery Research Grant 2023
PROJECT SUMMARY
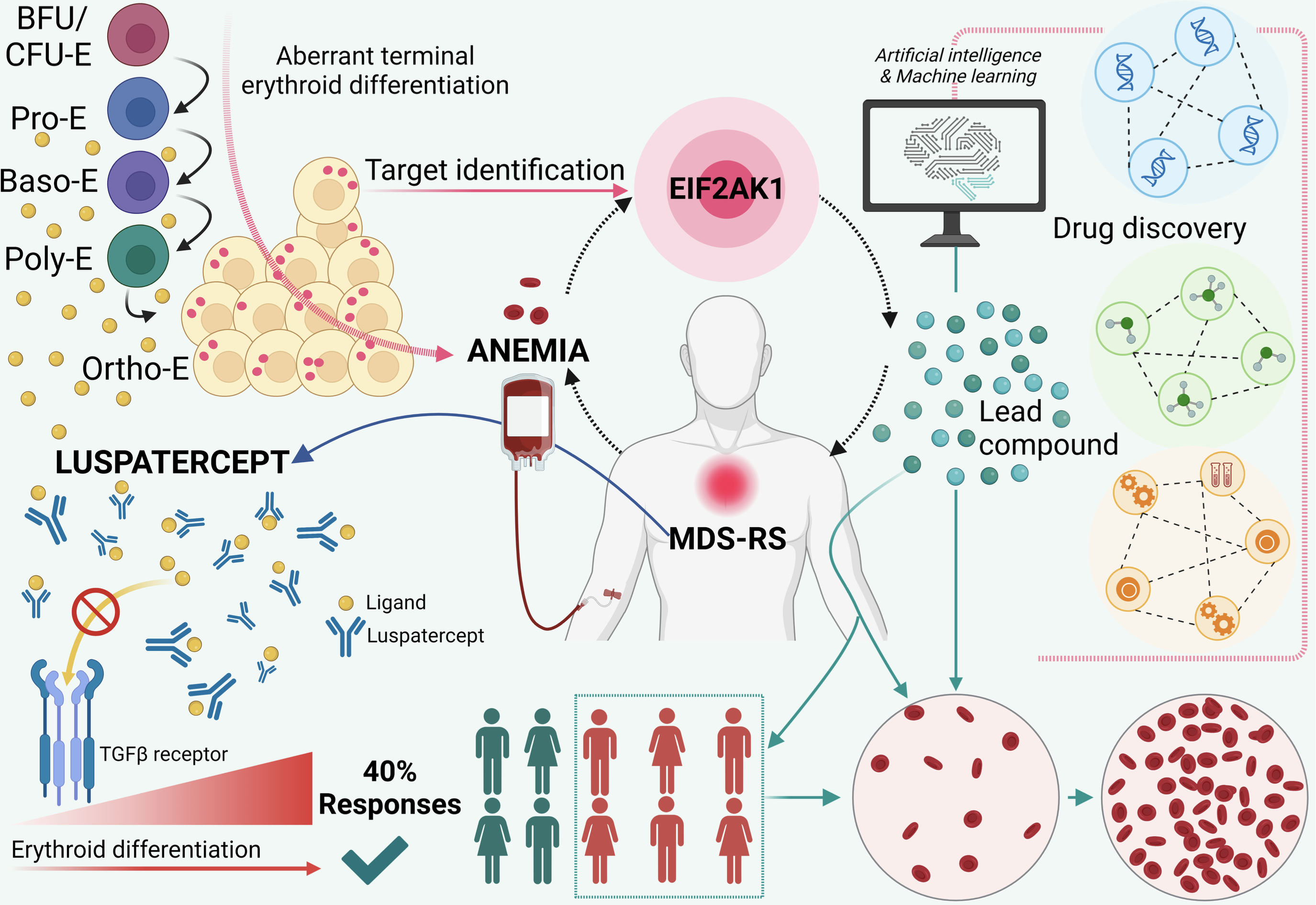
Myelodysplastic syndromes (MDS), which mainly affect older people, are blood cancers that prevent the body’s ability to produce blood cells, leading to symptoms such as anemia, bleeding, and infections. MDS are associated with relatively poor prognoses.
The most common form of MDS, which affects 20% of patients with MDS, is characterized by the formation of tumoral cells called ringed sideroblasts. Because of these cells, the disease is named MDS with ringed sideroblasts (MDS-RS). The cause of this type of MDS is an alteration in the DNA (called a mutation) that changes the function of a protein called SF3B1. MDS-RS has a low propensity for leukemic transformation, but patients with MDS-RS have severe anemia and require regular blood transfusions. Because of these continuous transfusions, patients with MDS-RS can die from iron overload in the liver and other adverse outcomes.
The only therapy approved for the treatment of MDS-RS patients who require transfusions and are ineligible for or have no response to drugs that stimulate red blood cell formation is luspatercept. However, luspatercept has a response rate of less than 40%, which underscores the need for alternative treatment options that can alleviate anemia in these patients.
In our previous study, we found that the EIF2AK1 protein is responsible for anemia in these patients. Therefore, we hypothesize that pharmacologically blocking EIF2AK1 can overcome aberrant red blood cell formation and thus anemia in patients with SF3B1-mutant MDS-RS. To test this hypothesis, we have partnered with the Institute of Applied Cancer Science at MD Anderson Cancer Center to develop a drug targeting this protein. Our multidisciplinary team has already identified several drugs targeting EIF2AK1.
In the proposed work, we will functionally validate these candidates using cellular assays (employing primary cells and induced pluripotent stem cells) that we have developed over the last 3 years. The successful completion of this study will enable the development of the first inhibitor of EIF2AK1 activity.
The proposed work has implications for the development of therapies to achieve long-lasting hematological responses in transfusion-dependent patients with MDS-RS. Given that EIF2AK1 inhibition is one of the most promising therapeutic approaches for sickle cell anemia to date, the results of our work may have a broad spectrum of clinical applications.