Researcher Profiles

Laura Calvi, M.D.
2020 Funding recipient
Role of facultative phagocytosis-induced metabolic disruption of mesenchymal stem cells in MDS progression
Discovery Research Grant 2020
PROJECT SUMMARY
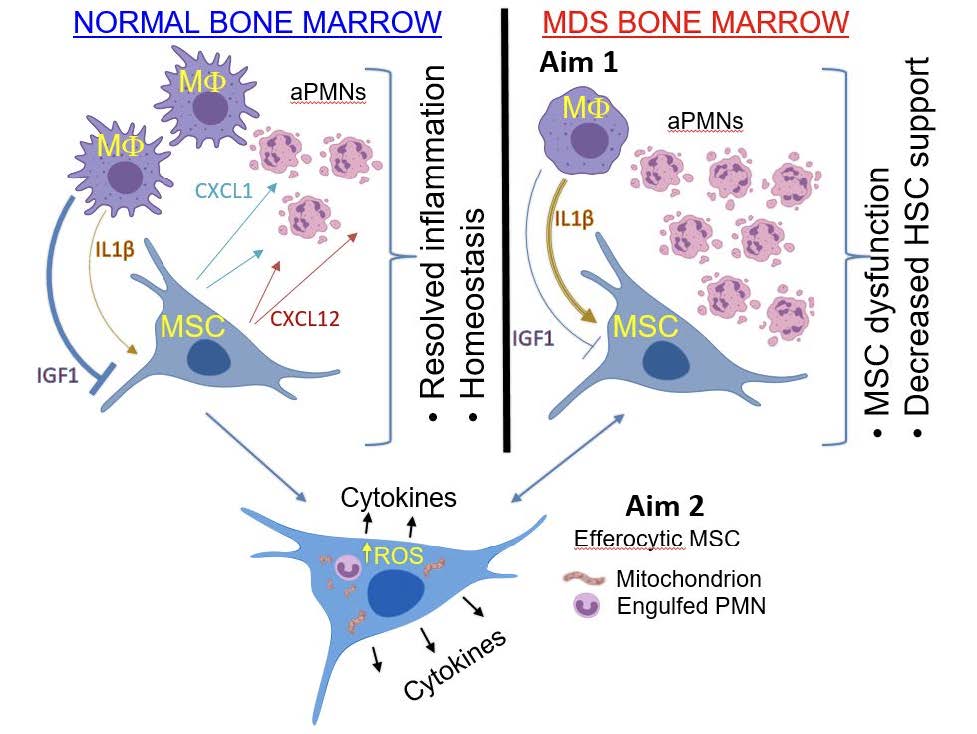
MDS resides in the bone marrow, where they interacts with the bone marrow microenvironment, which also supports normal blood stem cells. Many studies have shown that MDS is dependent on the microenvironment, which is abnormal and no longer supports normal blood stem cells. This work aims to understand a new mechanism that initiates dysfunction of the bone marrow microenvironment in MDS. We discovered that mesenchymal stromal cells, critical cells for the support of blood stem cells, contribute to the clearance of dead cells in the bone marrow. This activity is rare at baseline, but we have found that this activity is increased with aging and in MDS. During year 1, we identified defects in the engulfment of apoptotic cells by hematopoietic cells carrying the IDH2R172K mutation. The mutation was present in all differentiated cells derived from these mutant stem cells, including the bone marrow derived macrophages, which were defective in engulfing dying cells (efferocytosis). Over the past year, we have identified the specific mechanism that these microenvironmental cells use to engulf dying cells (the Axl and Tyro3 efferocytic receptors). Using a genetic model that genetically induces increased engulfment by MSCs, the cells have further increase in senescence. We have also identified IGF1 and Interleukin 1 from macrophages as key signals that regulate microenvironmental cells to do the engulfment. Importantly, we demonstrate that MSCs from mice with the IDH2R172K mutation have increased efferocytosis, are dysfunctional and have increased rates of senescence.
We have also demonstrated that acquisition of efferocytic activity by MSCs remodels their mitochondria, which is key to induce their loss of function. By identifying increased phagocytic activity by MSCs as a key consequence of MDS, given the increase in apoptotic cells in the marrow, combined with dysfunction of professional phagocytic cells, we are defining several novel therapeutic targets, including specific inhibition of MSC phagocytosis, enhanced macrophage phagocytosis, as well as metabolic changes initiated in MSCs, and in particular senescence of MSCs.