Researcher Profiles

Uma Borate, M.D., M.S.
2022 Funding recipient
Targeting the Inflammasome in high risk CH patients to prevent development of MDS/AML
Discovery Research Grant 2022
PROJECT SUMMARY
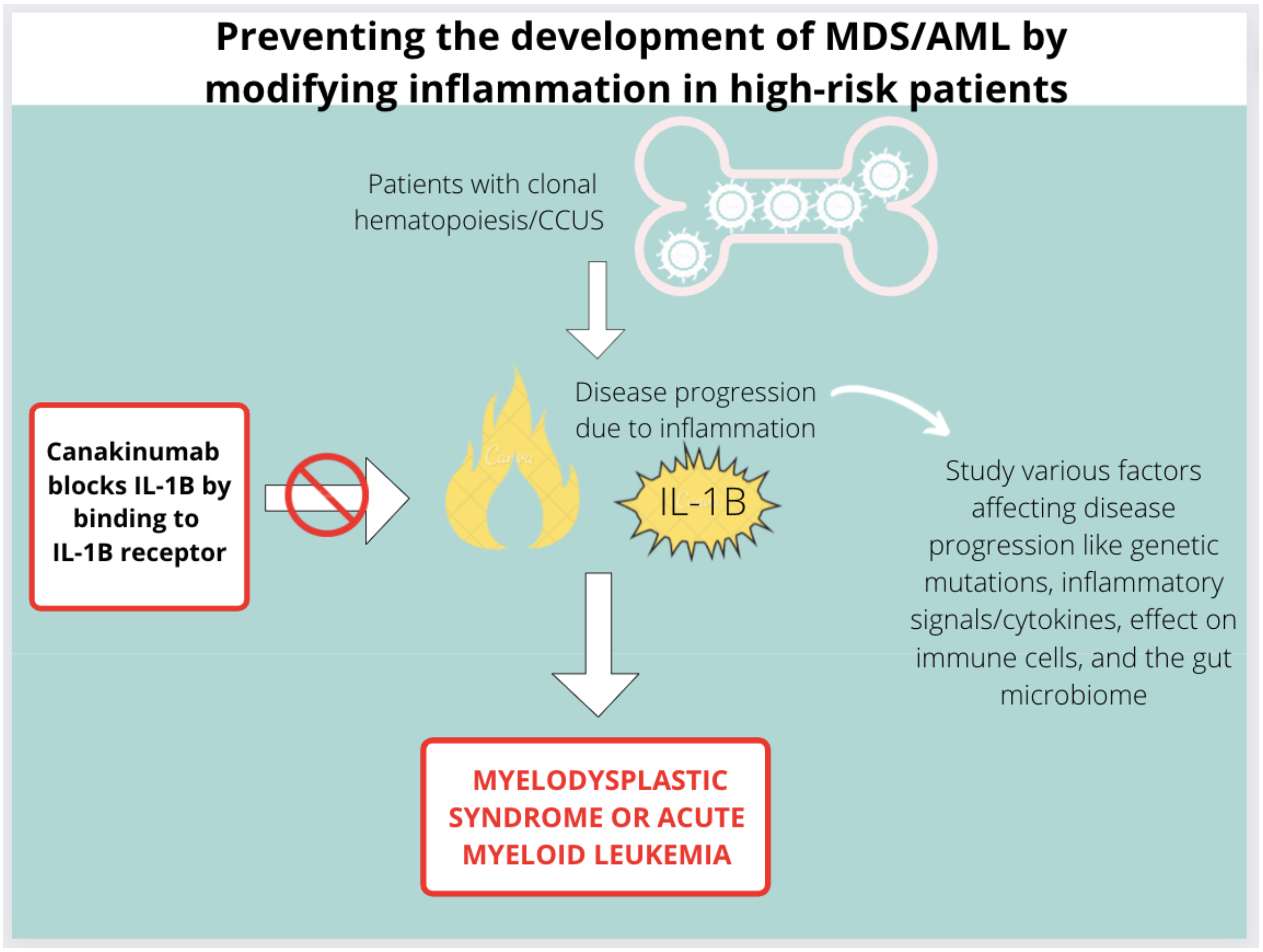
Our blood is made up of three types of blood cells: white blood cells, red blood cells, and platelets which arise from mother cells in our bone marrow, also known as stem cells. The stem cell undergoes a process called hematopoiesis in which it divides and matures into one of these three types of cells as it enters the blood. Occasionally during this division, a cell can acquire a mutation that causes it to live longer and divide differently. This group of cells, with a particular mutation, is called a clone and this process is called clonal hematopoiesis (CH). Over time, these clones can dominate the bone marrow, making it challenging for the bone marrow to make to make healthy blood cells. Eventually, the bone marrow can become dominated by these abnormal clones; eventually causing blood cancers such as Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML). People with low blood counts for more than four months also have CH and are diagnosed with Clonal Cytopenias of Undetermined Significance (CCUS). We still do not know why people develop CCUS or how to treat it. People with CCUS who have large clones and multiple genetic mutations, are 13 times more likely to develop bone marrow cancers or malignancies. Within five years, majority of people with high-risk CCUS will develop blood cancer. Older patients and patients with other cancers are more likely to have high-risk CCUS and are also more at risk of developing MDS or AML. When a patient is diagnosed with CCUS, they are usually observed with regular blood counts and there is no current treatment to slow the development of blood cancer.
Scientists have also shown that inflammation can worsen CH. Inflammation is a process in which the immune system releases various chemicals in response a stressor, such as infection. Cells communicate and warn other cells about these stressors by releasing messengers called cytokines. The cytokine that we think is important in driving inflammation in CH is called interleukin-1β (IL-1β). There has been extensive research that connects IL-1β with cancer development.
One such anti-inflammatory treatment is canakinumab, a monoclonal anti-human IL-1β antibody which is already approved for the treatment of inflammatory diseases by the Food and Drug Administration (FDA). Canakinumab blocks the interaction of IL-1β with the IL-1 receptor (IL-1R) by binding to human IL-1β. This stops the production of an inflammatory response (with other cytokines) that would otherwise worsen CH and promote the development of MDS or AML. In a large study, called the CANTOS trial, Canakinumab was shown to be effective in delaying progression of lung cancer by inhibiting IL-1β. Patients with low red blood counts (anemia) were found show improvement on this trial. This may suggest that this anti-inflammatory therapy may help prevent decreasing blood cell counts.
In our proposal, we aim to study the effects that canakinumab treatment will have on patients with CCUS who are participating in a Randomized Double-Blind Placebo-Controlled Phase II Multi- Center Study of Inflammation Modification of Canakinumab to Prevent Leukemic Progression of Clonal Cytopenias of Unknown Significance (CCUS) known as the IMPACT study. This trial is aimed at preventing blood cancer development in high risk CCUS patients. We will study the effects of this drug on CH, clone size, development of new mutations, different chemical messengers called cytokines, as well as our gut bacteria also called the microbiome. We think, that by targeting inflammation, we can alter the progression of CCUS and reduce the development of blood cancer; significantly improving patient outcomes. Our proposal provides the opportunity to study the specific effects of this treatment on the cellular, genomic, and immune biology of CH and will build a foundation for additional therapies for patients with this disease.