Researcher Profiles

Natthakan Thongon, Ph.D.
The University of Texas MD Anderson Cancer Center
2025 Funding Recipient
Dissect the molecular mechanisms of U2AF1-induced myelodysplastic syndromes in telomere biology disorders
EvansMDS Young Investigator Award 2025
PROJECT SUMMARY
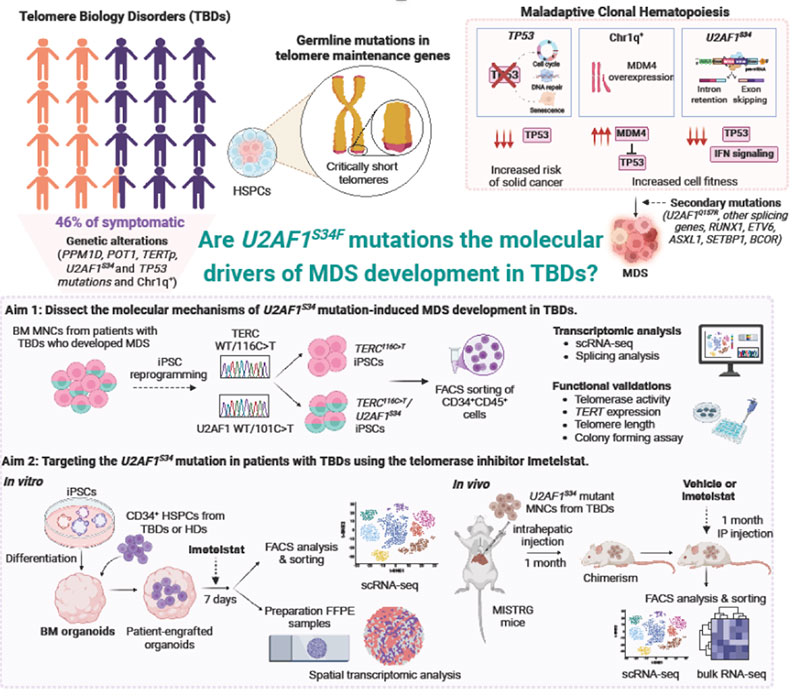
Telomeres are repetitive DNA sequences that play a crucial role in preventing chromosome loss when cells multiply. Telomere biology disorders (TBDs) are a group of diseases characterized by short telomeres induced by mutations in genes involved in telomere maintenance. Patients with TBDs may develop bone marrow (BM) failure and have a high risk of cancer, particularly myelodysplastic syndromes (MDS).
We studied over 200 patients with TBDs and found that genetic changes, including the U2AF1S34 and TP53 mutations and chromosome 1q gain, were strongly linked to MDS. Previous research explored how TP53 and chromosome 1q changes can affect blood stem cells when these cells’ telomeres are short. In preliminary findings, we showed that MDS development in the context of U2AF1S34 mutations allows cells to adapt to respond to genetic issues like the damage caused by short telomeres.
In this proposed work, I aim to improve our understanding of how U2AF1S34 mutations drive MDS progression in patients with TBDs and explore whether we can block the effect of these mutations using a drug called imetelstat, a telomerase inhibitor used in MDS treatment. I will use innovative models, including induced pluripotent stem cells and human BM organoids, to study the long-term effects of the drug and MDS progression over time. The proposed work will identify new potential treatments and useful genetic markers to guide the diagnosis and surveillance of TBD patients at high risk of developing MDS.