
Researcher Profiles

Jeffery M. Klco, M.D., Ph.D.
St. Jude Children's Research Hospital
2019 Funding recipient
Impact of SAMD9 and SAMD9L mutations on clonal evolution and fitness of hematopoietic stem cells
Discovery Research Grant 2019
PROJECT SUMMARY
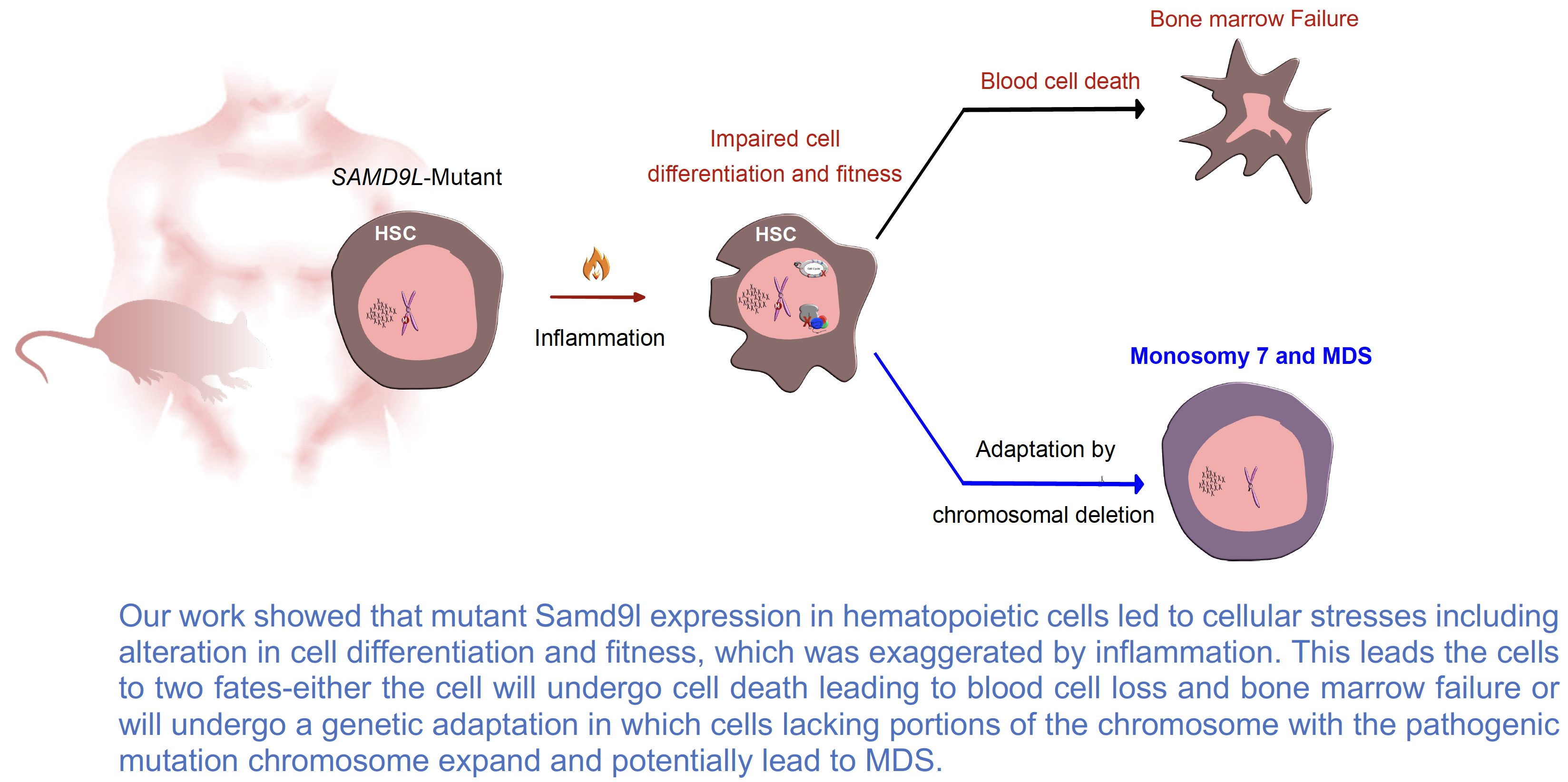
Our aim was to understand the contribution of germline mutations in SAMD9 and SAMD9L to normal blood cell formation and in the development of myelodysplastic syndromes (MDS). Patients with these mutations often suffer from decreased blood cells in the bone marrow and peripheral blood accompanied by fatigue, infections, and other complications. To release this pressure, mutant blood cells that lack the mutant allele, typically through copy number alterations, expand in the marrow but at the expense of potentially progressing to MDS due to the haploinsufficiency of chromosome 7 genes.
Over the last 36 months, we characterized our developed new conditional knockin mouse model that mimics the clinical features observed in patients. We found that cells from mutant Samd9l mice grow slower and are less fit than normal controls and these effects were furthered by inflammation. Using single-cell transcriptomics, we observed changes in the frequencies of blood cell subtypes with the most obvious suppression of B-lymphoid cells, which was also exaggerated with inflammation. Importantly, we observed non-random genetic deletion on chromosome 6 involving the Samd9l locus, equivalent to the chromosome 7 deletions seen in patients with myeloid neoplasms. These data were successfully published in The Journal of Clinical Investigation this year. Additionally, we have investigated the bone marrow cells of 12 patients at a single-cell level and got remarkable preliminary results that help us understand disease progression.
PUBLICATIONS
Jason R Schwartz, Jing Ma, Jennifer Kamens, Tamara Westover, Michael P Walsh, Samuel W Brady, J Robert Michael, Xiaolong Chen, Lindsey Montefiori, Guangchun Song, Gang Wu, Huiyun Wu, Cristyn Branstetter, Ryan Hiltenbrand, Michael F Walsh, Kim E Nichols, Jamie L Maciaszek, Yanling Liu, Priyadarshini Kumar, John Easton, Scott Newman, Jeffrey E Rubnitz, Charles G Mullighan, Stanley ,Pounds, Jinghui Zhang, Tanja Gruber, Xiaotu Ma, Jeffery M Klco, The acquisition of molecular drivers in pediatric therapy-related myeloid neoplasms, Nature Communications 2021 DOI: 10.1038/s41467-021-21255-8
Melvin E. Thomas III, Sherif Abdelhamed, Ryan Hiltenbrand, Jason R. Schwartz, Sadie Miki Sakurada, Michael Walsh, Guangchun Song, Jing Ma, Shondra M. Pruett-Miller & Jeffery M. Klco, Pediatric MDS and bone marrow failure-associated germline mutations in SAMD9 and SAMD9L impair multiple pathways in primary hematopoietic cells, Leukemia 2021 DOI: 10.1038/s41375-021-01212-6
Sherif Abdelhamed, Melvin E. Thomas III, Tamara Westover, Masayuki Umeda, Emily Xiong, Chandra Rolle, Michael P. Walsh, Huiyun Wu, Jason R. Schwartz, Virginia Valentine, Marcus Valentine, Stanley Pounds, Jing Ma, Laura J. Janke, and Jeffery M. Klco, Mutant Samd9l expression impairs hematopoiesis and induces bone marrow failure in mice, Journal of Clinical Investigation 2022, DOI: 10.1172/JCI158869
